Scientists have produced the natural pigment that gives octopuses, squids, and cuttlefish their color-shifting ability. Though the pigment, called xanthommatin, has interested researchers for many years due to its camouflage potential, it has previously been difficult to produce in labs.
Octopus Camouflage Technique
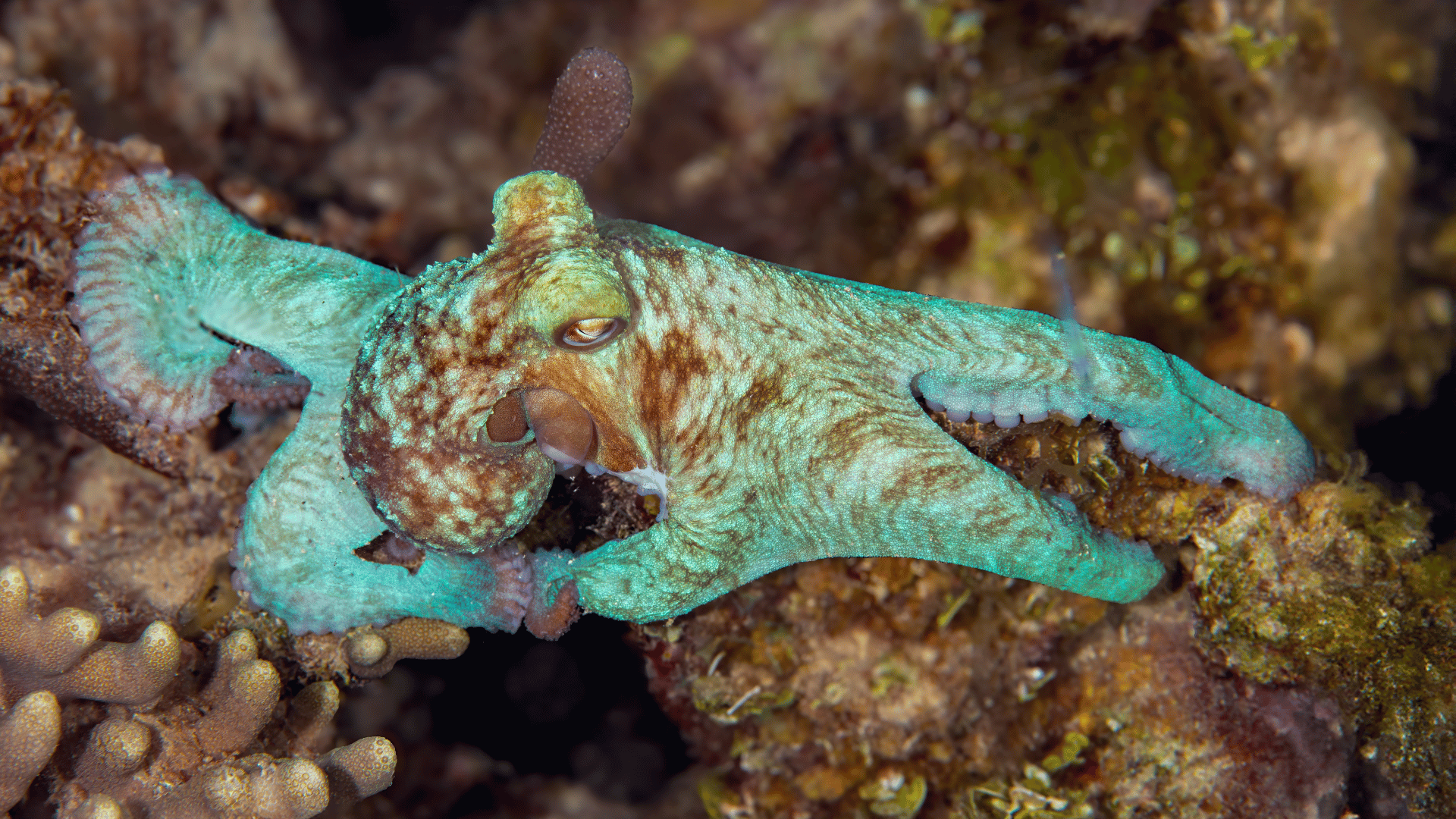

In addition to cephalopods, xanthommatin is also what gives monarch butterflies their orange tones or dragonflies their red coloring. The pigment has always been difficult to study, however, as it can only be extracted from animals in small amounts.
The new method was developed by researchers at the University of California, San Diego’s Scripps Institution of Oceanography. They discovered a new method for producing large quantities of xanthommatin using engineered bacteria.
Reportedly producing up to 1,000 times more pigment than traditional methods, marking a milestone in biomimicry.
“We’ve developed a new technique that has sped up our capabilities to make a material, in this case xanthommatin, in a bacterium for the first time,” noted Bradley Moore, senior author of the study and marine chemist at UC San Diego.
Advertisement
“This natural pigment is what gives an octopus or a squid its ability to camouflage — a fantastic superpower — and our achievement to advance production of this material is just the tip of the iceberg.”
The team invented a new biological system called “growth-coupled biosynthesis”, which connects the survival of bacteria to pigment production. This means the engineered bacteria won’t survive if they don’t produce the pigment. When the bacteria produce xanthommatin, they also generate formic acid, which fuels their own growth and creates a self-sustaining loop.
“We needed a whole new approach to address this problem,” stated Leah Bushin, lead author of the study and now a faculty member at Stanford University. “Essentially, we came up with a way to trick the bacteria into making more of the material that we needed.”
Researchers used robots and machine learning to evolve and optimize the bacteria. Moore and his team believe their new technique could revolutionize the production of biochemicals, providing a clean and efficient alternative to fossil fuel–based manufacturing.
“We’ve really disrupted the way that people think about how you engineer a cell,” remarked Moore in the press release.
“Our innovative technological approach sparked a huge leap in production capability. This new method solves a supply challenge and could now make this biomaterial much more broadly available.”
The study was published in Nature Biotechnology.