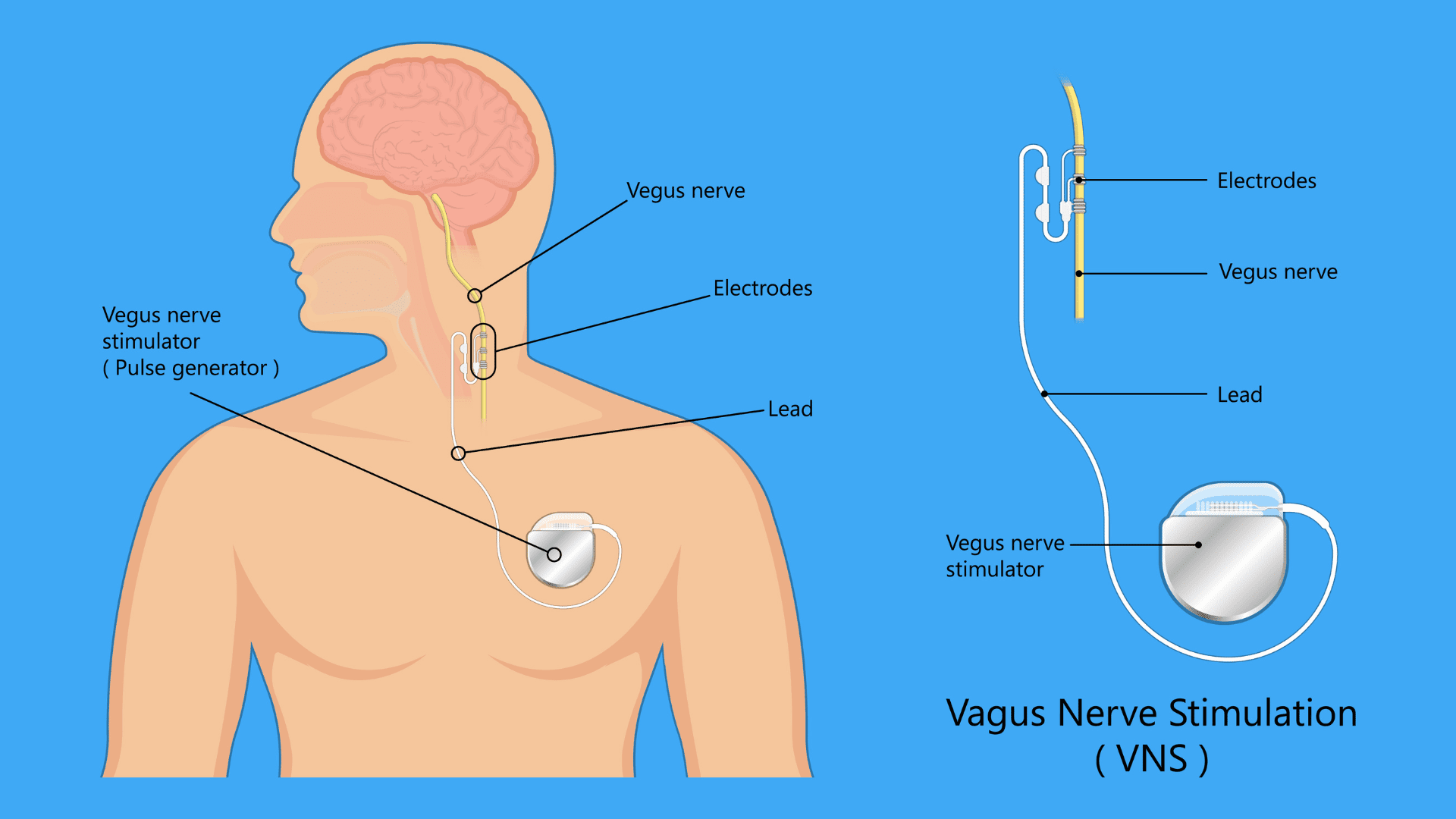
A pioneering medical device is creating buzz in the industry. The FDA recently approved the SetPoint system, a revolutionary implantable vagus nerve stimulator for treating rheumatoid arthritis (RA). This product results from decades of research by Dr. Kevin J. Tracey, president and CEO of the Feinstein Institutes for Medical Research.
This marks a significant leap in bioelectronic medicine and offers new hope for RA patients.
A Novel Implant Device

Dr. Tracey’s work at the Feinstein Institutes for Medical Research found a link between the vagus nerve and the body’s inflammatory response. He demonstrated that stimulating the nerve could effectively mitigate inflammation. The discovery laid the groundwork for the innovative device.
The device is called the SetPoint System. It’s a small device that is surgically implanted in the neck. The implant delivers one-minute electrical pulses to the vagus nerve daily. This small device is a rechargeable neurostimulator encased in a ceramic and titanium shell, which makes it both durable and biocompatible.
The SetPoint System is a novel treatment option for RA patients who have not yet found success with conventional therapies, such as biologic or targeted-synthetic DMARDs. It also operates as a personalized medicine tool. Rheumatology providers can determine automatic dosing through an iPad app, allowing them to tailor treatment to fit each patient’s specific needs. In addition, patients can conveniently recharge with a wireless charger.
One of the SetPoint System’s notable features is its ability to highlight a low rate of related serious adverse events. “There was a low rate (1.7%, n=4) of related serious adverse events in the peri-operative 3 months, with no (0%) related serious adverse events occurring through 12 months,” the report says.
Dr. Tracey believes this is just the beginning. He said, “We are close to a future where millions of patients will benefit from treatments like this — whether it’s for RA, epilepsy, depression, or other chronic conditions.”
Tune in to Science Channel to watch “Revolutionary Research” at 10 AM ET on Saturday, August 23rd!