Using Polluted water is a problem. The demand for Hydrogen production is high. A product to solve both of the issues is in the works from a team of researchers. It’s a product that turns polluted water into a fuel source.
Urea
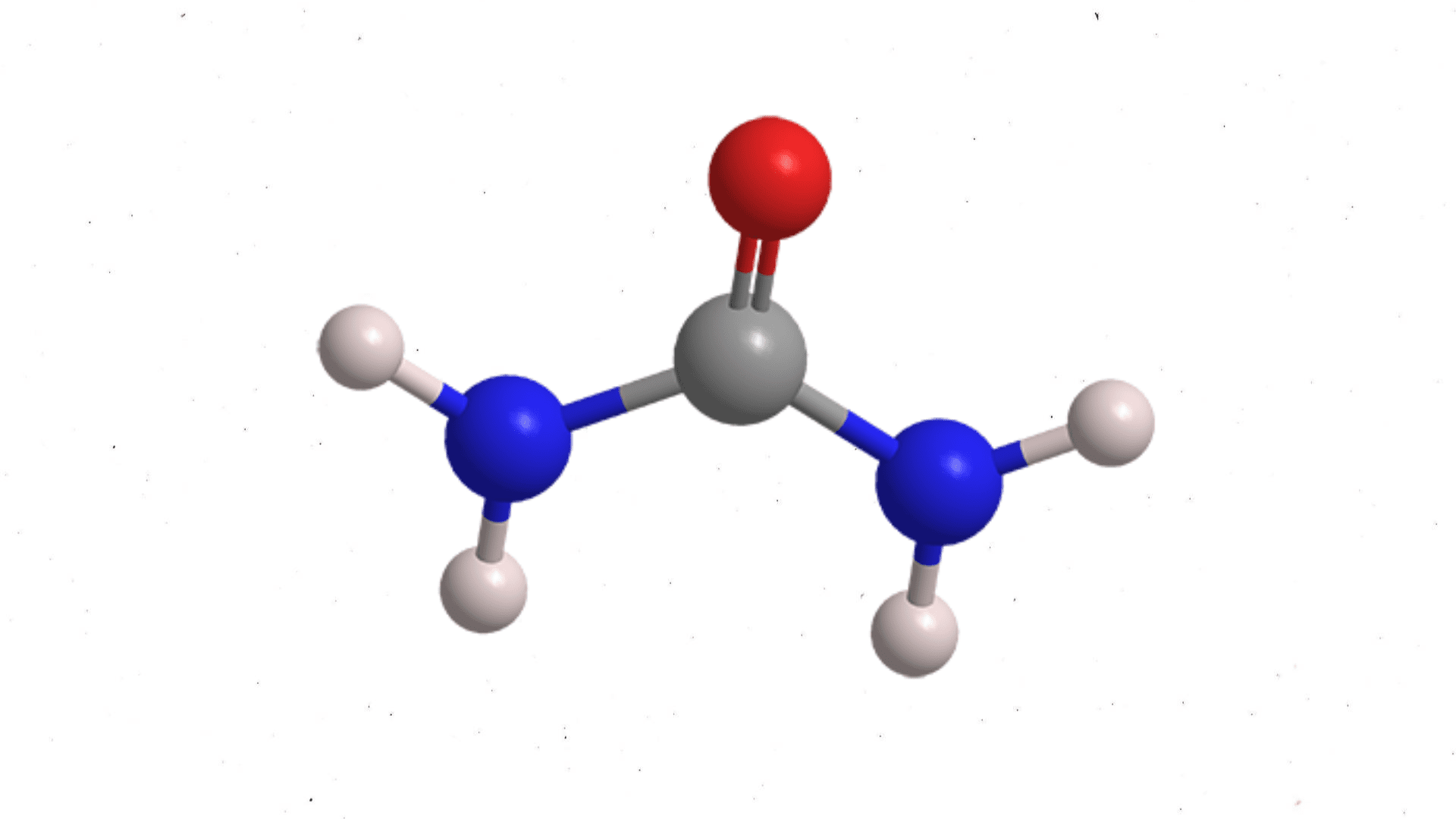
Let’s start with the issue. Urea is rich in nitrogen and a common agricultural fertilizer. It’s also a byproduct of human metabolism. That means it’s created in the body and extracted from it in different ways. When there is an abundance of urea in an excessive amount of water it poses challenges to the environment. Wastewater discharge rich with urea affects aquatic environments and human health because it contributes to algal blooms and hypoxic dead zones. Researchers from the Worcester Polytechnic Institute (WPI) is taking a significant step to remove the urea from water and turn it into hydrogen gas.
Explore Tomorrow's World from your inbox
Get the latest science, technology, and sustainability content delivered to your inbox.
I understand that by providing my email address, I agree to receive emails from Tomorrow's World Today. I understand that I may opt out of receiving such communications at any time.
A few things make urea a good candidate to make hydrogen fuel. While it can be dangerous, it’s non-toxic with significant hydrogen content and it’s able to dissolve in water easily to make a solution.
Using urea for fuel

There is a weakness in utilizing urea for hydrogen production. There is a lack of low-cost and highly efficient electrocatalysts, or elements used to create electricity, that successfully oxidize urea instead of water. Xiaowei Teng and his team of researchers at WPI address this issue in their study. Teng and his team create and use electrocatalysts composed of nickel and cobalt atoms that positively interact with each other. Focus is on nickel and cobalt oxides and hydroxides in the study. Researchers figured out when they tailors unique electronic structures with dominant nickel and cobalt species, it enhances electrochemical activity and selectivity for urea oxidation.
Computer simulations conducted by Professor Aaron Deskins supports the findings. It shows that mixing nickel and cobalt oxides and hydroxides facilitates electron redistribution. What this does is optimizes the substances for bonding with urea and water molecules. The findings revolutionize how urea is used. Not only could it efficiently produce hydrogen fuel but it could also contribute to the long-term sustainability of ecological systems.
The research opens new possibilities for the water-energy nexus, providing an environmentally friendly approach to both water treatment and hydrogen production.