Hydrogen is central to future clean energy options for a sustainable, fossil-free world. However, hydrogen embrittlement threatens its widespread use. This issue weakens metals used in hydrogen-powered systems, including high-pressure vessels and pipelines.
Researchers have been unable to determine the exact atomic-scale mechanisms behind this obstacle because the element is difficult to detect within solid materials.
In a groundbreaking experiment, researchers from the University of Oxford and Brookhaven National Laboratory observed how hydrogen impacts stainless steel from the inside.
Researchers utilized a specialized X-ray imaging technique to monitor how minor defects in the steel, called dislocations, react to the presence of hydrogen. Lead researcher Dr. David Yang said, “For the first time, we have directly observed how hydrogen changes the way defects in stainless steel behave deep inside the metal, under realistic conditions.”
A Groundbreaking Experiment for Future Hydrogen Use
Dr. Yang noted that this insight is crucial for developing alloys durable enough to withstand harsh environments, such as those in future hydrogen-powered aircraft and nuclear fusion plants.
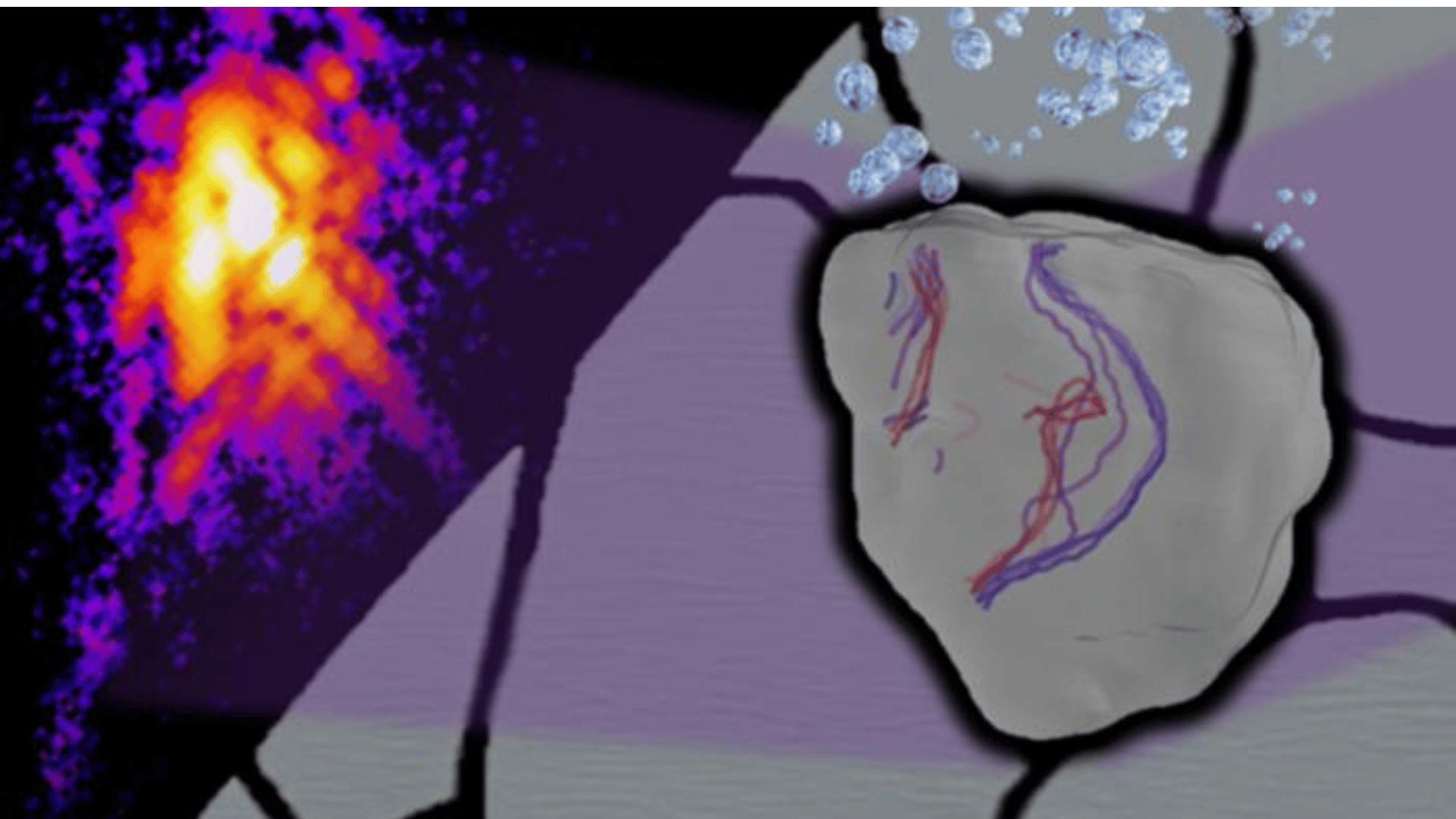
The team carried out the study using a nondestructive method known as Bragg Coherent Diffraction Imaging at a powerful U.S. facility. This allowed them to visualize a single stainless-steel grain in real time over 12 hours.
“Using coherent X-ray diffraction, a nondestructive method, we were able to watch atomic-scale events unfold in real time inside solid metal without cutting open the sample,” said Felix Hofmann, a University of Oxford professor and the study’s lead investigator. “It has been tremendously exciting analysing this data and piecing together the parts of this scientific puzzle.”
The results surprised the team and showed them unexpected behavior.
One of the three surprising findings was that the dislocations in the metal became unexpectedly mobile. According to the team, the dislocations moved and reshaped without external stress, indicating that hydrogen acts like a lubricant.
Another surprise was the defects’ unusual “out-of-plane” or “climb” motion. They say this motion is not usually seen at room temperature, suggesting that hydrogen allows atoms to rearrange in ways that can make alloys softer.
Finally, researchers found the first direct experimental measurement of a long theorized effect called hydrogen elastic shielding. They found that the area of distortion, or the strain field, was noticeably reduced as hydrogen built up. This helps the researchers understand why hydrogen leads to unexpected failures by allowing internal defects to move more freely and in untraditional ways.
According to the researchers, this work directly influences how to model and predict the behavior of materials in hydrogen environments. It points toward possible strategies for creating new alloys with greater resistance to hydrogen embrittlement.